Professional use only
Read carefully all the information in this manual before using the product. Save this manual for future reference.
Product description:
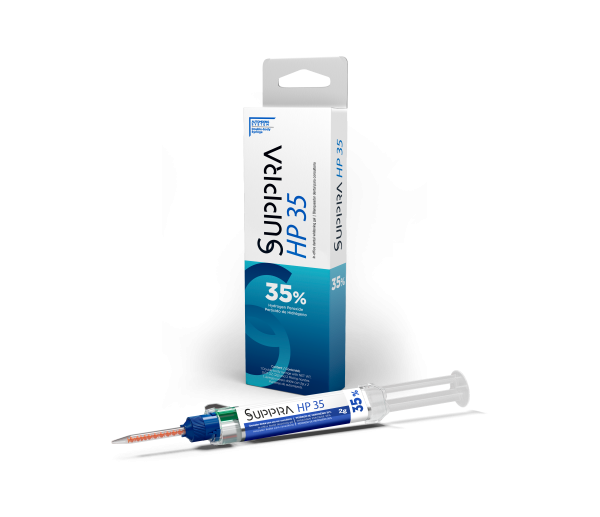
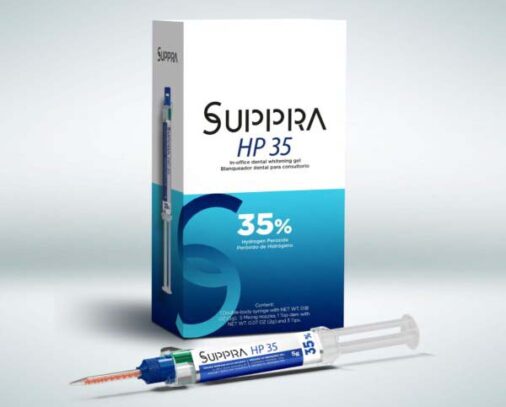
SUPPRA HP35 is a 35% hydrogen peroxide whitening gel for vital teeth. It should be used exclusively at a dental office. Always use gingival tissue isolation for proper safety. The product is presented in a doublebody auto-mixing syringe containing peroxide and thickener material in different compartments. The system uses special tips that mix the two materials automatically delivering a ready-to-use gel with excellent viscosity. For the whitening procedure, the use of external light sources is optional. If a light source is used, Suppra HP 35 contains a heat blocking agent. Another advantage of the gel is that it contains calcium which prevents the enamel from demineralization during whitening process. The gel is colored which helps identify the areas the gel has been applied to.
Presentation;
Kit: 1 double-body syringe with NET WT. 0.18 OZ (5g) of whitening gel, 5 auto-mixing nozzles,
Refill: 1 syringe with NET WT. 0.07 OZ (2g) of gingival barrier (Suppra Dam), 3 applicator tips.
Ingredients:
water (aqua), hydrogen peroxide, polyethylene glycol, silicon dioxide, triethanolamine, calcium gluconate, chromium hydroxide green (CI 77289).
Product Indication:
Whitening of vital teeth exclusively at the dental office. It can be used with or without light sources.
Precautions and Contraindications:
· Do not use the product on patients with a known sensitivity to peroxides, glycols, acrylates, other resins or any of the components of the formula. If allergic reaction, dermatitis or rash develops, stop using and consult a physician.
· The product is exclusive for in-office use and should be used by dentists only.
· The health of the oral cavity should be evaluated before starting treatment. Caries, pulp alterations, gingival or periodontal disease, cracks or fissures in enamel or dentin, restorations presenting marginal leakage, exposed dentin and other factors that can compromise the whitening treatment should be fixed before beginning.
· The product is not indicated for whitening teeth with amelogenesis and dentinogenesis imperfecta, severe fluorosis, intense staining by tetracycline and other enamel and dentin anomalies, which may put the vitality of the teeth at risk.
· The product is not recommended for use under anesthesia. It is important to monitor the sensitivity felt by the patient during the whitening process. In extreme cases when there are imperfections or failures in the tooth structure in such a way that the peroxide can get to the dental pulp, there is a risk of pulp necrosis.
· The product is not recommended for lactating or pregnant women and patients under 15 years of age.
· The product is not recommended for patients who have undergone recent gingival surgery or patients showing inflammatory condition of the gingival tissue.
· The product is not recommended for use with high-power lasers and others that result in high temperatures on the tooth surface. The temperature of the dental pulp should not surpass 42ºC (108ºF) to avoid irreversible damage.
· Acid etching of the dental enamel before whitening is neither necessary nor recommended.
· While using the product, professionals as well as their assistants should wear protective gloves and goggles. Patients should also use the necessary protection to avoid accidental contact of the product with skin or clothes.
· Efficiently isolate gingival tissue using a lightcuring gingival barrier such as Suppra Dam and a lip retractor. It is important to avoid the contact of the peroxide with soft tissues.
· Suppra HP 35 is a whitening product with high oxidizing power. If in contact to soft tissues, it can cause chemical burning on different levels: from mild irritation with local redness to white spots,
ulceration, edema and pain. These signs usually disappear within 2 hours without any aftereffect, but depending on the duration of the contact and on the physiological characteristics of the tissue,
injuries can be expected. If the gel has contact with the soft tissue, remove the gel with a saliva ejector, rinse off and apply a peroxide neutralizing solution immediately by rubbing the area with a
cotton pellet soaked with the solution. Depending on the severity of the burning, discontinue the whitening session. Follow up with your patient until there are no more signs of injury and seek
medical care if needed.
· The whitening gel come in a syringe with a double-body that contains both materials in different compartments: the peroxide and the thickener. When removing the closing cap of the syringe, one should be careful not to push the plunger to avoid product from spilling.
· The content of the syringe (peroxide thickener) may be under pressure and/or may have leaked due to adverse storage conditions. Before opening the package, protect your hands and eyes and
check for leakage inside the package. In case leakage is identified, please request product replacement.
· Set up the product far from the patient, over a sink or safe counter, without the risk of contaminating anything or person.
· After using the product, wash your hands and any peroxide material that might have been discharged. When using the product, always have prevention and safety in mind.
· Patients should be warned to avoid ingesting acid foods and/or strongly pigmented foods for at least 24 hours after the whitening treatment to prevent sensitivity and possibly compromising the
process.
Side Effects
· Dental hypersensitivity may occur during or after whitening. Because, in several scenarios, hypersensitivity is hard to predict, the use of a desensitizing agent is recommended as a prophylactic desensitizing procedure before whitening. If hypersensitivity persists and it is not possible to continue the treatment, schedule a new appointment with a minimum interval of 7 days. Assess if there are no anomalies in the teeth that can be corrected to eliminate hypersensitivity.
· There might be cases of high levels of hypersensitivity after the procedure (1 or 2 hours later). In those cases, prescribing analgesics and applying desensitizing agents is recommended.
· The whitening treatment may highlight preexisting spots of dental hypocalcification due to a more intense whitening effect of those areas. Sometimes, the spots were invisible before treatment and then become more evident as the treatment goes on. In this case, it is suggested to pause the treatment and apply sodium fluoride daily, until the spots are gone. If it persists, discuss with the patient to consider stopping the treatment since the progression of those spots is uncertain.
Instructions for use
Preparing for whitening:
1. Remove the product from its packaging observing the safety recommendations. When using the syringe, wear disposable gloves.
2. Remove the closing cap from the double-body syringe. Then, firmly attach the auto-mixing nozzle to the syringe, paying attention that the coupling is correct. Press the plunger gently until the product is released from the tip with a consistent gel making sure this is done away from the patient (over a sink or counter). A small amount of unmixed product may be released at first during mixing process, simply discard it.
3.Verify if the product is flowing correctly from the syringe before applying to the teeth. Any resistance should be checked. Make sure the nozzle is correctly attached to the syringe and if needed, change the applicator nozzle.
Note: The product should be used at room temperature. If stored in a refrigerator, leave the package at room temperature for at least 30 minutes before beginning the treatment.
Whitening vital teeth
Make a good assessment of the patient’s oral cavity. The presence of caries, deficient restorations and fissures in the enamel, gingival recessions, gingivitis and other important characteristics should be verified and treated before proceeding. Provide protection for yourself and the patient. Using prophylactic brush and pumice and desensitizing agents is recommended before beginning the treatment. This will reduce the chance of dental hypersensitivity throughout the process.
Step by Step
1. Proceed with the prophylaxis of the teeth and leave the dental surface to be whitened free from biofilm. Register the shade of the patient’s teeth by means of a shade guide and/or photograph before beginning the treatment.
2. Proceed with inserting a lip retractor to facilitate access and manipulation in the buccal cavity. Apply a desensitizing agent on the teeth to be whitened before starting.
3. Remove the desensitizing gel with water spray and dry the gingival tissue. Apply the gingival barrier Suppra Dam on the gingival margins with a layer that is 3mm to 5mm wide and 1mm thick.
The barrier should cover approximately 0.5mm to 1 mm of the tooth surface and also any areas of exposed dentin. Use an intraoral mirror observing from incisal to cervical to see if there is uncovered gingival tissue. If so, make sure to add more Suppra Dam. This stage is crucial to avoid contact of the peroxide with the gingiva. Take special care on papilla areas. After application, light cure the Suppra Dam resin with 20 to 30 seconds of curing for each group of 3 teeth, using a scanning motion. The gingival protector that is formed is rigid and insoluble to preventing irritation.
4. With the auto-mixing nozzle correctly coupled to the double-body syringe, press the plunger gently until the two materials (peroxide and thickener) are slowly mixed in the tip. A small amount of gel should be disposed in a container before applying the product. That will guarantee that the product applied to the teeth is correctly homogenized. Refer to the section “Preparing for whitening” presented in this document.
5. Apply the gel directly on the teeth to be whitened, normally from the 1st or 2nd premolar to the opposing 1st or 2nd premolar. Cover the buccal face of the teeth with the thinnest possible gel layer, using the auto-mixing nozzle only to spread the material. The covering with gel may be extended to the small area of the incisal/occlusal surface of the teeth. In case you want to use a light source, begin using it right after applying the gel.
Each light source equipment has a specific time protocol for light exposure. Follow the instructions of the manufacturer to confirm the duration of the light application.
6. After the application, the gel should be left in contact with the teeth for up to 50 minutes. During that time, the professional should move the gel with an explorer or disposable micro applicator to remove any air voids to renew the contact of the gel to the teeth. There is no need to re-apply a new layer of gel during the session.
7. At the end of the session, remove the gel with a fine ejector and wash thoroughly. The removal of the gingival barrier may be carried out with an explorer or similar instrument.
8. It is recommended to polish teeth with felt disks and polishing paste after the session.
9. Office appointments should be scheduled with intervals of 7 days; allowing for effective evaluation of tooth shade transition. If necessary, more whitening sessions may be scheduled according to the steps described above. In general, 2 or 3 in-office bleaching sessions are suggested to get the best results.
Important Notes
1. To prevent occasional dental hypersensitivity during or after the treatment, desensitizing agents can be applied before the whitening treatment, in a prophylactic way. Applying it after the whitening treatment is optional. In cases of severe post session hypersensitivity, desensitizing agents can be applied daily in dental tray by the patient until the symptoms disappear.
2. Monitor patients throughout the entire process for hypersensitivity and possible peroxide irritated areas of soft the tissue. Fissures in the enamel or dentin exposure can lead to acute and intense pain. In those cases, suspension of the process and the search for corrective treatment before resuming whitening are recommended.
3. If the results obtained with the whitening procedure do not fulfill the patients’ expectations and the patient does not produce hypersensitivity or any other contraindication, a re-application of the product may be performed for two more sessions, with a total of three sessions. If more sessions are needed, a minimum interval of 7 days is necessary.
Additional Information
Overview:
· Like with the other whitening techniques, the following steps are recommended: register the shade of the teeth before treatment. Explain the possible outcome of the case to the patient (limitations in the case of staining by tetracycline, grey coloration, etc.). Inform the patient about possible hypersensitivity and the eventual need for restoration replacement. There are cases in which, due to certain characteristics of the teeth (enamel characteristics, pigmentation type, etc.), the desired level of whitening may not be possible.
· For most cases, 2 or 3 sessions are enough for a complete whitening treatment.
· In case the patient develops hypersensitivity that cannot be controlled, an alternative may be the reduction of the duration of each individual whitening session. In this case, the professional should verify if there are not any anomalies in the tooth structure that could be causing the hypersensitivity (fissures, exposed dentin, etc.).
When the hypersensitivity is difficult to control or for some reason a new whitening session is neither possible nor recommended, the treatment can be complemented with the use of a take-home
whitener.
About the gel
· Upon mixing the product, it produces a green color, which does not change during whitening.
· Double – body syringes should be store with the tip tightly closed. The used auto-mixing nozzles should be disposed after each session.
· The product was designed so that each doublebody syringe provides up to 8 applications from 2nd premolar to the opposing 2nd premolar, on one arch. Considering that the whitening will be carried out on both arches, the product will provide up to 4 complete sessions. However, sometimes that number of applications cannot be reached due to differences on the size of the teeth or quantity of applied gel. For maximum economy (without losing performance) it is recommended to apply the thinnest layer of gel possible to cover all the buccal surfaces of teeth to be whitened.
· Unused whitening gel inside the syringe (non mixed content) can be stored and used according to the storage and expiry data on the package and in this “Instruction for Use” document.
· The application nozzles are designed for single use only.
About the light device
In case a light source is used during the whitening procedure, care should be taken to avoid overheating the teeth by the device. Each device should come with its usage and safety protocol. Read carefully the instructions of the equipment and compare them to the information provided by the product Suppra HP 35. Follow the instructions to make the process as safe as possible.
Conservation and Storage
· Make sure the cap of the double-body syringe with the remaining material is tightly closed.
· Store the product at a location with temperatures between 5°C and 25°C / 41°F and 77°F for the best conservation. Do not freeze the product. Protect it from direct sunlight and humidity.
Warnings
· Do not use this product if it is expired. For disposing the product, check your country’s disposal requirements.
· Keep out of the reach of children. This product has been manufactured for dental purposes and should be used according to the instructions for use contained in this document.
The manufacturer will not be held responsible for any damage caused by using this product for a different purpose or by incorrect usage. It is the obligation and responsibility of the dental professional to verify before using, this material that it is compatible to the desired application, mainly when the use is not recommended in this instructions for use document. Data descriptions do not constitute any type of warranty and therefore, are not legally binding.
SUPPRA HP35 dental professionals can enjoy efficiency and practicality in a single convenient product. This hydrogen peroxide teeth whitening gel is made using a proprietary formulation to ensure successful whitening for patients looking to improve their smiles and confidence.
Hydrogen peroxide teeth whitening is regarded as the most effective form of in-office whitening. Suppra pioneered in teeth whitening product and continues to bring innovations to the market today, with all of our whitening products now available in the United States.
Features
- Self-mixing system.
- Single application per session.
- Contains calcium.
- 35% hydrogen peroxide teeth whitening gel.
Product indications
Whitening of vital and non-vital teeth using the in-office technique with 35% hydrogen peroxide teeth whitening gel.
Easy handling and great results from a hydrogen peroxide teeth whitening gel kit
Suppra HP 35 is packaged in a convenient and innovative syringe applicator that self-mixes to reduce the time and complexity of preparing a whitening solution. The neutral pH improves patient safety and the efficacy of the whitening gel. Viscous and suitable for contact of up to 50 minutes, this advanced hydrogen peroxide teeth whitening gel actively works throughout the entire session, ensuring that results are swift and noticeable. The blue-green color tint remains constant throughout the whitening treatment to ensure visibility and maximum control for the clinician.
Suppra HP 35 helps to streamline dental practice costs thanks to a resealing syringe. Clinicians can use as much or as little as they need for the specific patient application. Syringes can be resealed and stored for future use.
With little risk of hypersensitivity thanks to a heat blocking formulation, even when using operative lights, this is the best choice for discerning dentists who want to deliver the absolute best hydrogen peroxide teeth whitening service to their patients.
Packing containing OR Order info:
SUP 1001 SUPPRA HP 35 KIT,
- 1 double-body syringe with 5g of whitening gel,
- 5 self-mixing applicator tips,
- 1 syringe with 2g of gingival barrier (SUPPRA Dam),
- 3 Applicators and Instruction for use.
SUP 1002 SUPPRA HP 35 Refill,
- 1 double-body syringe with 2g of whitening gel,
- 2 self-mixing applicator tips, and Instruction for use.
SUP 1003 SUPPRA Self-Mixx 48 Applicators tips








Medova
A device for measuring blood pressure is a medical device that helps individuals monitor their blood pressure levels at home.
Medova
A device for pressure measuring blood is a medical device that helps individuals monitor their blood pressure levels at home.
Medova
A device for pressure measuring blood is a medical device that helps individuals monitor pressure levels at home their blood .
Medova
A device for device that pressure measuring blood is a medical helps individuals monitor pressure levels at home their blood .